Authors: Mai Ping Tan, PhD and Elizabeth Harvey, PhD, members of the MedCommsTech Medical Writers Collective

The Academy of Managed Care Pharmacy (AMCP) Format for Formulary Submissions is a key resource for market access and value communications professionals, setting out guidance for formulary dossier submissions in the United States. The latest updates to the AMCP Format, released in version 5.0 on March 31, 2024, are of interest not only for market access and value communications specialists, but also for the wider medical communications community. The updates reflect key topics in healthcare: digital therapeutics, health disparities, pre-approval information exchange, real-world evidence, and the need for brevity in communications.1,2
We review the main updates in the AMCP Format v5.0 and their broader applicability in medical and value communications.
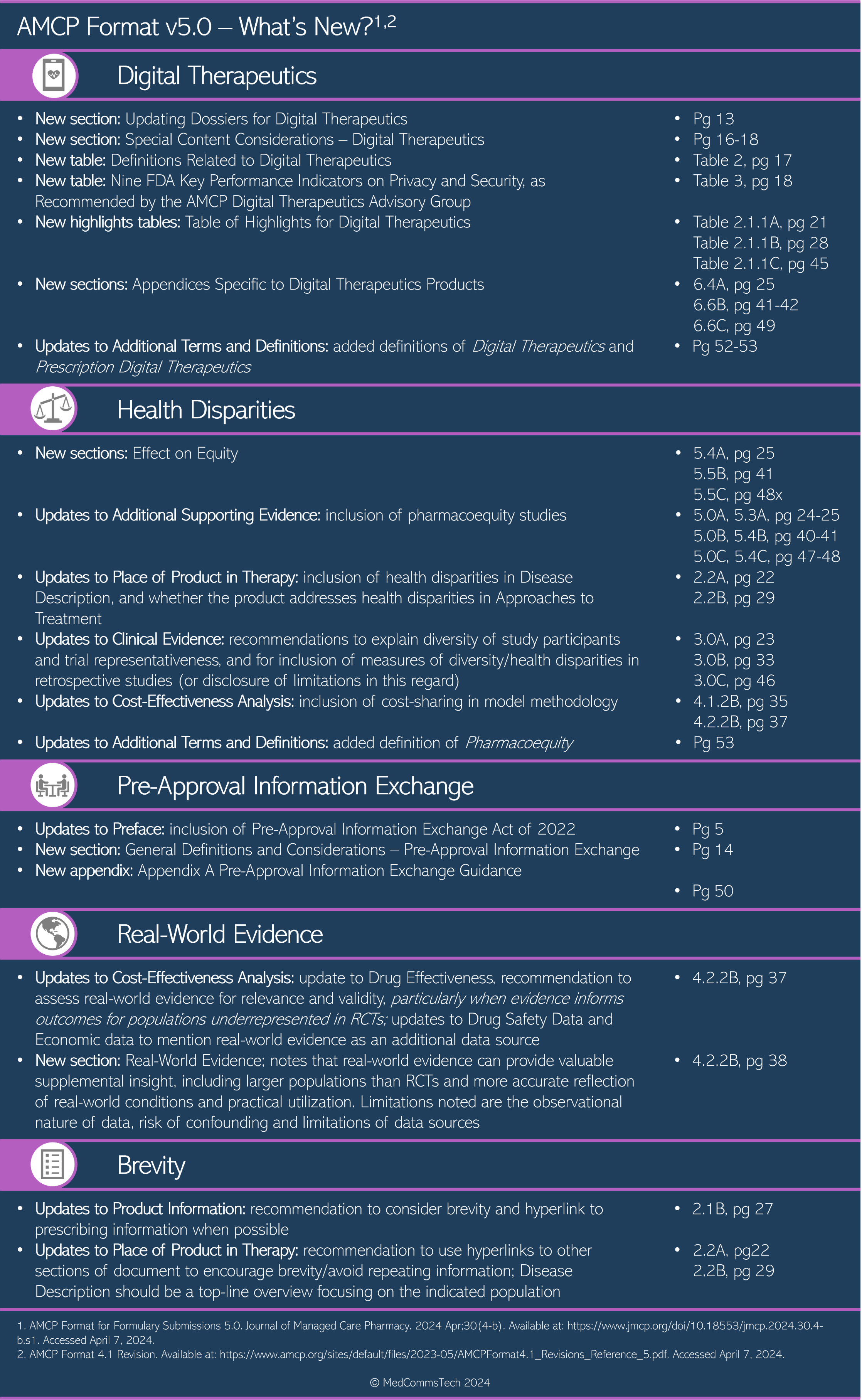
Digital therapeutics
As increasing numbers of digital therapeutics (software, applications or programs that help to prevent or manage disease) become available, they bring new considerations for product evaluation and reporting, including data security, privacy, compatibility with other technologies, availability of technical assistance, and management of releases and updates. The AMCP Format v5.0 aims to standardize the communication of evidence to allow healthcare decision makers to evaluate digital therapeutics for healthcare coverage and reimbursement. As such, medical communication professionals may also use the AMCP Format as a resource in communications relating to the evaluation and reporting of evidence on novel digital therapeutics.
Health disparities
Addressing health disparities is a critical need across all areas of healthcare. Updates to the AMCP Format include guidance on reporting diversity and representativeness of clinical trials and retrospective studies, and evaluations of pharmacoequity. The AMCP Format defines pharmacoequity as ensuring that all individuals, regardless of race and ethnicity, socioeconomic status, or resource availability, have access to the highest-quality medical therapy required to manage their health needs. This goal should be at the forefront across medical and value communications.
Pre-approval information exchange
Following the 2022 passage of the Pre-approval Information Exchange (PIE) Act in the United States, the updated AMCP Format formalizes recommendations and guidance for PIE communication tools in a new appendix. Medical and value communications professionals can consider referencing the appendix as a checklist when developing PIE materials. AMCP recommends including information on ongoing and completed trials, timelines for FDA submission, expected approval and launch, and other information relevant to patient access, such as limited or restricted distribution plans.
Real-world evidence
The latest updates extend guidance on incorporating real-world evidence in value assessments, and recommend considering real-world evidence particularly for populations underrepresented in clinical trials. AMCP notes that real-world evidence provides valuable data about practical utilization of therapies, often representing larger numbers of patients than in clinical trials, while encouraging manufacturers to be transparent about study and data source limitations. Because real-world evidence may be used to inform many aspects in health and disease (e.g., epidemiology, burden of illness, treatment outcomes), this principle applies throughout medical and value communications.
Brevity
The updated AMCP Format encourages brevity without sacrificing clarity, with practical recommendations such as using hyperlinks to other sections of the dossier to avoid repetition, and reducing the recommended number of pages for the place of the product in therapy. The goal of brevity is often more easily set than achieved, but this is an area where experienced medical and value communications professionals can make all the difference!
For more details on the updates in the AMCP Format v5.0, see our summary table below.
Summary of AMCP Format v5.0 updates1,2
References
- AMCP Format for Formulary Submissions 5.0. Journal of Managed Care Pharmacy. 2024 Apr;30(4-b). Available at: https://www.jmcp.org/doi/10.18553/jmcp.2024.30.4-b.s1. Accessed April 7, 2024.
- AMCP Format 4.1 Revision. Available at: https://www.amcp.org/sites/default/files/2023-05/AMCPFormat4.1_Revisions_Reference_5.pdf. Accessed April 7, 2024.
Mai Ping Tan and Elizabeth Harvey are freelance medical writers and members of MedCommsTech Medical Writers Collective. The opinions expressed in this article are the authors’ own and have not been reviewed or endorsed by AMCP.